Medical Devices
Research group - Mechatronic assistance for patient’s benefit
Motivation
The research group Surgical Assistance at the Institute of Micro Technology and Medical Device Technology (MIMED) examines the efficiency of medical mechatronic systems. In various projects, we develop devices and systems to assist surgeons or to directly benefiting the patients. For this purpose, we are working in the field of mechanics, electronics and information technology. Particularly, the potentialities of rapid manufacturing (e.g. selective laser sintering) are systematically explored.
The systems and devices are developed and certified according to the current standards of medical devices, because our objective is the proof of our concepts with clinical trials and evaluation. We take care, that our devices show a benefit for the physician and the patient in the real clinical environment.
Methodology
OR-ready mechatronic systems
The rising complexity of medical procedures creates the need for support systems for surgeons during interventions. Mechatronic or mechanical systems can provide physical assistance as well as information during surgery.
The use of prototypes in the operating room require high security standards during the development and production. Several prototypes developed in our group has already been utilized in the operating room following standards such as DIN EN ISO 60601 or DIN EN ISO 62304.


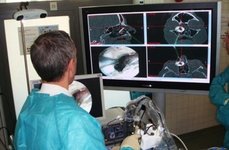
Planning and intraoperative navigation of surgical interventions
Especially in minimally-invasive surgical procedures planning and intraoperative navigation of the surgical intervention and the imaging of the anatomy is crucial as the operation site is not openly accessible and the physician has to rely on information provided on the screen. Therefore, our aim is the development of planning and navigation systems to support the planning and performing procedure and so improve surgical outcome.

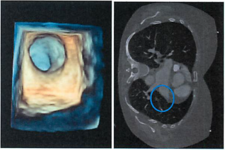
Functional anatomic models
Models are an essential part of the clinical evaluation and the training of physicians. Functional models do not only imitate the shape of anatomic structures but also specific function, such as sound transmission. These models can be used for functional experiments such as the verification of sound transmission of stapes prosthesis.
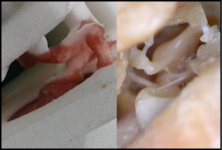
Medical requirement specification - Clinical prototype evaluation
Understanding clinical problems and the physicians’ perspective on medical needs is essential for the success of developing medical devices. The cooperation with clinical partners starts long before the beginning of the development and continues during the whole project. Thereby it is ensured, that the development of novel devices solves existing medical problems. The aim of every project is the clinical trial and evaluation of prototypes.
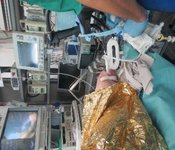
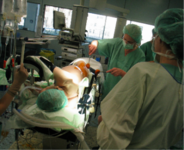
Projects
Facilities
- X-ray shielded operating room inside MIMED with OR equipment
- Medical device show room
- Steam sterilizer
- Others

Selected Publications
- Coemert, S.; Kollmer, M.; Olmeda, M.; Krieger, Y. S.; Brecht, S. V.; Lueth, T. C. (2017): Development of a Double Arm Endoscopic Mini-Manipulator System for Transurethral Resection of Bladder Tumors (TURBT). 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24.09. - 28.09.2017, pp. 1670-1676.
- Coemert, S.; Traeger, M. F.; Graf, E. C.; Lueth, T. C. (2017): Suitability evaluation of various manufacturing technologies for the development of surgical snake-like manipulators from metals based on flexure hinges. Procedia CIRP, 65, pp. 1-6. DOI: 10.1016/j.procir.2017.03.108
- Pfeiffer, J. H.; Moser, T. F.; Dietz, C.; Krieger, Y. S.; Lueth, T. C. (2017): Distributed Navigated Control for Active Instruments in a Real-Time Networked Operating Room. 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24.09. - 28.09.2017, pp. 1-6.
- Brecht, S.V.; Krieger, Y.S.; Stolzenburg, J.-U.; Lueth, T.C. (2016): A New Concept for a Single Incision Laparoscopic Manipulator System Integrating Intraoperative Laparoscopic Ultrasound. IEEE International Conference on Robotics and Biomimetics (Robio), Qingdao, China, December 03-07, 2016. DOI: 10.1109/ROBIO.2016.7866296
- Lueddemann, T.; Sahin, S.; Pfeiffer, J.; Lueth, T.C. (2016): Experimental Evaluation of a Novel ISO 14971 Risk Management Software for Medical Devices. International Symposium on System Integration, Sapporo, Japan, 13.-15.12.2016. DOI: 10.1109/SII.2016.7843992
- Pfeiffer, J.; Borbáth Á.; Dietz, C.; Lueth T.C. (2016): A New Module Combining Two Tracking Cameras to Expand the Workspace of Surgical Navigation Systems. International Symposium on System Integration, Sapporo, Japan, 13.-15.12.2016. DOI: 10.1109/SII.2016.7844044
- Graf, E.C.; Tiemann, K.; Praceus, J.; Lüth T.C. (2016): A Planning System of the Implant Size and Position for Minimally-Invasive Closure of the Left Atrial Appendage. IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics, Singapore, 26-29.06.2016, pp. 293-298. DOI: 10.1109/BIOROB.2016.7523641
- Lueddemann, T.; Chang, D.; Sahin, S.; Lueth, T.C. (2016): Medical Device Approval Process in China since the Introduction of the China Food and Drug Administration. IEEE Symposium on Product Compliance Engineering (ISPCE), Anaheim, USA, May 16-18, 2016. DOI: 10.1109/ISPCE.2016.7492842
- Kuru, I.; Maier, H.; Müller, M.; Lenarz, T.; Lueth, T.C. (2016): A 3D-printed functioning anatomical human middle ear model. Hearing Research, 304, pp. 204-213. DOI: 10.1016/j.heares.2015.12.025, PMID: 26772730
- Kuru, I.; Roth, M.; Ferrer, F.L.; Krieger, Y.S.; Lenarz, T.; Maier, H.; Lueth, T.C. (2016): Design and Manufacturing Concept of a Postoperative Adjustable Prosthesis for Ossicular Chain Reconstructions. IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics, Singapore, 26-29.06.2016, pp. 1-6. DOI: 10.1109/BIOROB.2016.7523767